Next we must understand state or phase change.
Every time H20 changes phase from solid to liquid or gas, it either absorbs (Endothermic) or releases (Exothermic) what’s known as ‘latent heat’. The values vary depending on source and other
conditions such as pressure but generally at atmospheric pressure the following applies
Melting: absorbs 330,000 J/kg of latent heat
Evaporation: absorbs 2,500,000 J/kg latent heat
Sublimation: absorbs 2,830,000 J/kg latent heat
Freezing: releases 330,000 J/kg latent heat
Deposition: releases 2,830,000 J/kg latent heat
Condensation: releases 2,500,000 J/kg latent heat
Now if we can create condensing within a boiler heat exchanger, that energy, a potential 2,500,000 Joules per litre of H20 can be absorbed as heat back in to the heating water.
So how do we maximise condensation?
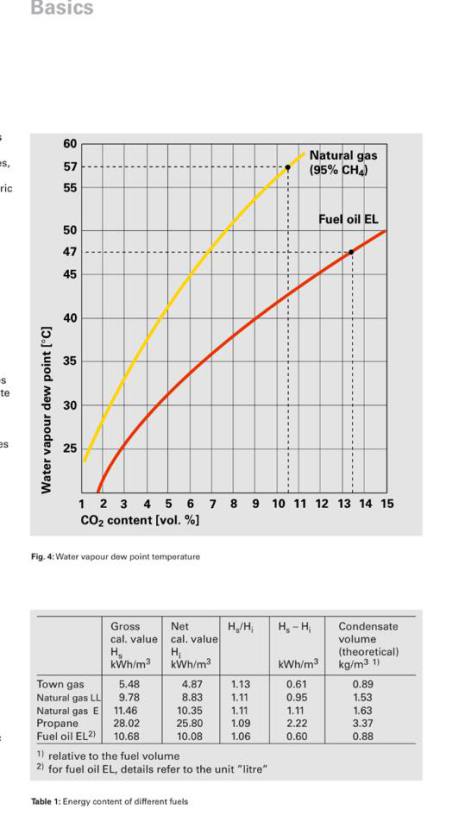
Condensation occurs when humid air comes in contact with a cooler surface, the maximum temperature of the surface before condensing will no longer occur is known as the dew point, and will depend on
2 variables, pressure and humidity. Since the pressure differences inside our heat exchangers are negligible the only one of concern is humidity.
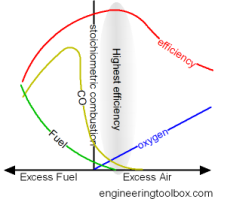
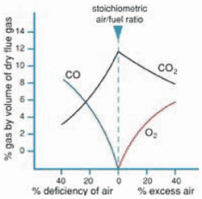
Now because we know that our main POC are CO2 and H20, and their concentration is only varied by the combustion mix of excess air and our gas fuel source, buy measuring one we will have an indication
of the other. That is to say, the higher the CO2 content the higher the H2O or humidity.
The higher this humidity the higher the temperature that condensing can occur as displayed here.
So the ideal is to have a return temperature as low as possible and below this dew point. If we can also get our flow temperature below this point we will have the maximum surface area within the
heat exchanger possible for the condensing to occur.
However there is a caveat! When we get condensing occurring the humidity drops, this in turn lowers our dew point (we want as high as possible to extract more latent heat) and
slows or halts the condensing process… so if for example we had a very large heat exchanger, huge infact, a dew point of 55, and our return temperature was 54, you may suppose we could eventually
exchange all the vapour in to water and extract all the latent heat, when in actual fact as soon as the humidity drops slightly the dew point decreases and condensing will theoretically halt. It is
for this reason that it is it is essential to use compensation controls which will attempt to run the system, and in turn the return temperature to the boiler as low as possible below the dew point,
not ‘just below it’, if you want to extract the maximum amount of latent heat. the further below dew point, the more condensing that will occur, latent heat absorbed and the more energy saved.
In fact research commissioned by Viessmann and conducted independently by the University of Salford recently found that connecting a boiler to weather compensation controls will reduce energy
consumption by 15% when the outside temperature is 3°C, by 31% when it is 8°C outdoors, and by 45% when it is 12°C outside, due to having lower return temps. Details on this study are hard to find
but a sensible conclusion would be that larger radiators will have similar effect. The over all savings from this study where 15% due to hot water production.
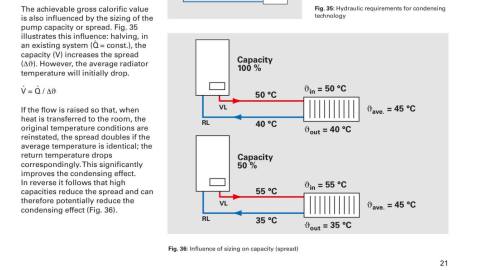
This is also the reason it is generally accepted as ideal to run natural gas domestic boilers at a delta T of 20oc. The following diagram, again from Viessmann displays this.
There are many more reasons that low temperatures add efficiency, for example the larger the differential between the flame temperature to heat exchange the more efficient the heat transfer,
slower corrosion rates, and gentler on heating system components/prolonged life of the system. To maximise the abortion of this otherwise wasted heat and the other benefits described an advanced
weather compensated control will provide the lowest possible flow temperatures. Contact your local Ecotechnician for guidance on how this may be achieved on your system.